Kornelite
A valid IMA mineral species - grandfathered
This page is currently not sponsored. Click here to sponsor this page.
About Kornelite
Formula:
Fe2(SO4)3 · 7H2O
Colour:
Pale rose pink to violet.
Lustre:
Silky
Specific Gravity:
2.306
Crystal System:
Monoclinic
Name:
For Kornel Hlavacsek (1835-1914), Hungarian mining engineer at the pyrite mines, Banska Stavnica, Slovakia.
Type Locality:
This page provides mineralogical data about Kornelite.
Unique Identifiers
Mindat ID:
2253
Long-form identifier:
mindat:1:1:2253:4
GUID
(UUID V4):
(UUID V4):
453f6794-85f4-4517-9a74-6f52b6693ce7
IMA Classification of Kornelite
Approved, 'Grandfathered' (first described prior to 1959)
IMA Formula:
Fe3+2(SO4)3 · 7H2O (?)
First published:
1888
Type description reference:
Classification of Kornelite
7.CB.60
7 : SULFATES (selenates, tellurates, chromates, molybdates, wolframates)
C : Sulfates (selenates, etc.) without additional anions, with H2O
B : With only medium-sized cations
7 : SULFATES (selenates, tellurates, chromates, molybdates, wolframates)
C : Sulfates (selenates, etc.) without additional anions, with H2O
B : With only medium-sized cations
29.8.2.1
29 : HYDRATED ACID AND NORMAL SULFATES
8 : A2(XO4)3·H2O
29 : HYDRATED ACID AND NORMAL SULFATES
8 : A2(XO4)3·H2O
25.10.8
25 : Sulphates
10 : Sulphates of Fe alone
25 : Sulphates
10 : Sulphates of Fe alone
Mineral Symbols
As of 2021 there are now IMA–CNMNC approved mineral symbols (abbreviations) for each mineral species, useful for tables and diagrams.
| Symbol | Source | Reference |
|---|---|---|
| Knl | IMA–CNMNC | Warr, L.N. (2021). IMA–CNMNC approved mineral symbols. Mineralogical Magazine, 85(3), 291-320. doi:10.1180/mgm.2021.43 |
Physical Properties of Kornelite
Silky
Transparency:
Transparent
Comment:
If fibrous
Colour:
Pale rose pink to violet.
Streak:
White
Cleavage:
Distinct/Good
On {100}
On {100}
Density:
2.306 g/cm3 (Measured) 2.254 g/cm3 (Calculated)
Optical Data of Kornelite
Type:
Biaxial (+)
RI values:
nα = 1.572 nβ = 1.586 nγ = 1.640
2V:
Measured: 49° to 62°, Calculated: 56°
Max Birefringence:
δ = 0.068

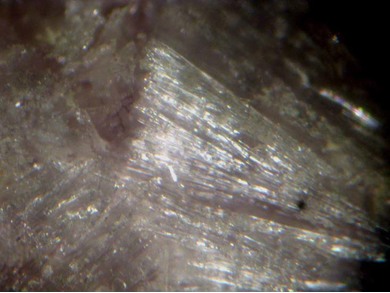
Image shows birefringence interference colour range (at 30µm thickness)
and does not take into account mineral colouration.
and does not take into account mineral colouration.
Surface Relief:
Moderate
Dispersion:
very weak r>v
Chemistry of Kornelite
Mindat Formula:
Fe2(SO4)3 · 7H2O
Elements listed:
Crystallography of Kornelite
Crystal System:
Monoclinic
Class (H-M):
2/m - Prismatic
Space Group:
P21/m
Cell Parameters:
a = 14.3(1) Å, b = 20.12(2) Å, c = 5.425(4) Å
β = 96.8(1)°
β = 96.8(1)°
Ratio:
a:b:c = 0.711 : 1 : 0.27
Unit Cell V:
1,549.88 ų (Calculated from Unit Cell)
Z:
4
Morphology:
Crystals lath-like {010} and acicular [001]. Crusts, tufted aggregates, globular masses with a radial-fibrous structure.
Twinning:
Polysynthetic on {100}.
Crystal Structure
Load
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Unit Cell | Unit Cell Packed
2x2x2 | 3x3x3 | 4x4x4
Show
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Big Balls | Small Balls | Just Balls | Spacefill
Polyhedra Off | Si Polyhedra | All Polyhedra
Remove metal-metal sticks
Display Options
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
Black Background | White Background
Perspective On | Perspective Off
2D | Stereo | Red-Blue | Red-Cyan
View
CIF File Best | x | y | z | a | b | c
CIF File Best | x | y | z | a | b | c
Rotation
Stop | Start
Stop | Start
Labels
Console Off | On | Grey | Yellow
Console Off | On | Grey | Yellow
Data courtesy of the American Mineralogist Crystal Structure Database. Click on an AMCSD ID to view structure
| ID | Species | Reference | Link | Year | Locality | Pressure (GPa) | Temp (K) |
|---|---|---|---|---|---|---|---|
| 0000322 | Kornelite | Robinson P D, Fang J H (1973) Crystal structures and mineral chemistry of hydrated ferric sulphates. III. The crystal structure of kornelite American Mineralogist 58 535-539 |  | 1973 | 0 | 293 |
CIF Raw Data - click here to close
X-Ray Powder Diffraction
Powder Diffraction Data:
| d-spacing | Intensity |
|---|---|
| 10.0 Å | (FFF) |
| 6.64 Å | (FF) |
| 4.67 Å | (FF) |
| 4.30 Å | (F) |
| 3.14 Å | (F) |
| 4.39 Å | (mF) |
| 3.50 Å | (mF) |
Geological Environment
Paragenetic Mode(s):
| Paragenetic Mode | Earliest Age (Ga) |
|---|---|
| Stage 7: Great Oxidation Event | <2.4 |
| 47a : [Near-surface hydration of prior minerals] | |
| 47b : [Sulfates and sulfites] | |
| Stage 10b: Anthropogenic minerals | <10 Ka |
| 55 : Anthropogenic mine minerals |
Type Occurrence of Kornelite
Place of Conservation of Type Material:
Natural History Museum, Budapest, Hungary. Type specimen destroyed 1956, topotypes remain.
Geological Setting of Type Material:
Weathering product in a pyrite mine
Associated Minerals at Type Locality:
Reference:
Krenner, J. (1888) Huszonötödik akadémiai ülés. Magyar Tudományos Akadémia Értesítöje: 22: 131-131.
Other Language Names for Kornelite
Common Associates
Associated Minerals Based on Photo Data:
| 2 photos of Kornelite associated with Copiapite | Fe2+Fe3+4(SO4)6(OH)2 · 20H2O |
| 2 photos of Kornelite associated with Szomolnokite | FeSO4 · H2O |
| 1 photo of Kornelite associated with Paracoquimbite | Fe4(SO4)6(H2O)12 · 6H2O |
| 1 photo of Kornelite associated with Jarosite | KFe3+3(SO4)2(OH)6 |
| 1 photo of Kornelite associated with Römerite | Fe2+Fe3+2(SO4)4 · 14H2O |
| 1 photo of Kornelite associated with Coquimbite | AlFe3(SO4)6(H2O)12 · 6H2O |
Related Minerals - Strunz-mindat Grouping
| 7.CB. | Sarvodaite | Al2(SO4)3 · 5H2O |
| 7.CB.02 | Voudourisite | CdSO4 · H2O |
| 7.CB.05 | Dwornikite | Ni(SO4) · H2O |
| 7.CB.05 | Gunningite | ZnSO4 · H2O |
| 7.CB.05 | Kieserite | MgSO4 · H2O |
| 7.CB.05 | Poitevinite | (Cu,Fe)SO4 · H2O |
| 7.CB.05 | Szmikite | MnSO4 · H2O |
| 7.CB.05 | Szomolnokite | FeSO4 · H2O |
| 7.CB.05 | Cobaltkieserite | CoSO4 · H2O |
| 7.CB.07 | Sanderite | MgSO4 · 2H2O |
| 7.CB.10 | Bonattite | CuSO4 · 3H2O |
| 7.CB.12 | Belogubite | CuZn(SO4)2 · 10H2O |
| 7.CB.15 | Aplowite | (Co,Mn,Ni)SO4 · 4H2O |
| 7.CB.15 | Boyleite | (Zn,Mg)SO4 · 4H2O |
| 7.CB.15 | Ilesite | (Mn,Zn,Fe)SO4 · 4H2O |
| 7.CB.15 | Rozenite | FeSO4 · 4H2O |
| 7.CB.15 | Starkeyite | MgSO4 · 4H2O |
| 7.CB.15 | Drobecite | CdSO4 · 4H2O |
| 7.CB.15 | Cranswickite | MgSO4 · 4H2O |
| 7.CB.20 | Chalcanthite | CuSO4 · 5H2O |
| 7.CB.20 | Jôkokuite | MnSO4 · 5H2O |
| 7.CB.20 | Pentahydrite | MgSO4 · 5H2O |
| 7.CB.20 | Siderotil | FeSO4 · 5H2O |
| 7.CB.25 | Bianchite | Zn(SO4) · 6H2O |
| 7.CB.25 | Chvaleticeite | Mn(SO4) · 6H2O |
| 7.CB.25 | Ferrohexahydrite | FeSO4 · 6H2O |
| 7.CB.25 | Hexahydrite | MgSO4 · 6H2O |
| 7.CB.25 | Moorhouseite | Co(SO4) · 6H2O |
| 7.CB.25 | Nickelhexahydrite | Ni(SO4) · 6H2O |
| 7.CB.30 | Retgersite | NiSO4 · 6H2O |
| 7.CB.35 | Bieberite | CoSO4 · 7H2O |
| 7.CB.35 | Boothite | CuSO4 · 7H2O |
| 7.CB.35 | Mallardite | MnSO4 · 7H2O |
| 7.CB.35 | Melanterite | Fe2+(H2O)6SO4 · H2O |
| 7.CB.35 | Zincmelanterite | (Zn,Cu,Fe)SO4 · 7H2O |
| 7.CB.35 | Alpersite | (Mg,Cu)(SO4) · 7H2O |
| 7.CB.40 | Epsomite | MgSO4 · 7H2O |
| 7.CB.40 | Goslarite | ZnSO4 · 7H2O |
| 7.CB.40 | Morenosite | NiSO4 · 7H2O |
| 7.CB.45 | Alunogen | Al2(SO4)3 · 17H2O |
| 7.CB.45 | Meta-alunogen | Al2(SO4)3 · 12H2O |
| 7.CB.50 | Aluminocoquimbite | Al2Fe2(SO4)6(H2O)12 · 6H2O |
| 7.CB.50 | Lazaridisite | 3CdSO4 · 8H2O |
| 7.CB.52 | Pararaisaite | CuMg[Te6+O4(OH)2] · 6H2O |
| 7.CB.55 | Coquimbite | AlFe3(SO4)6(H2O)12 · 6H2O |
| 7.CB.55 | Paracoquimbite | Fe4(SO4)6(H2O)12 · 6H2O |
| 7.CB.55 | Rhomboclase | (H5O2)Fe3+(SO4)2 · 2H2O |
| 7.CB.55 | Raisaite | CuMg[Te6+O4(OH)2] · 6H2O |
| 7.CB.57 | Caichengyunite | Fe2+3Al2(SO4)6 · 30H2O |
| 7.CB.65 | Quenstedtite | Fe2(SO4)3 · 11H2O |
| 7.CB.70 | Lausenite | Fe2(SO4)3 · 5H2O |
| 7.CB.75 | Lishizhenite | ZnFe2(SO4)4 · 14H2O |
| 7.CB.75 | Römerite | Fe2+Fe3+2(SO4)4 · 14H2O |
| 7.CB.80 | Ransomite | CuFe2(SO4)4 · 6H2O |
| 7.CB.85 | Apjohnite | Mn2+Al2(SO4)4 · 22H2O |
| 7.CB.85 | Bílinite | Fe2+Fe3+2(SO4)4 · 22H2O |
| 7.CB.85 | Dietrichite | (Zn,Fe2+,Mn2+)Al2(SO4)4 · 22H2O |
| 7.CB.85 | Halotrichite | FeAl2(SO4)4 · 22H2O |
| 7.CB.85 | Pickeringite | MgAl2(SO4)4 · 22H2O |
| 7.CB.85 | Redingtonite | (Fe2+,Mg,Ni)(Cr,Al)2(SO4)4 · 22H2O |
| 7.CB.85 | Wupatkiite | (Co,Mg,Ni)Al2(SO4)4 · 22H2O |
| 7.CB.90 | Meridianiite | MgSO4 · 11H2O |
Other Information
Notes:
Water soluble
Health Risks:
No information on health risks for this material has been entered into the database. You should always treat mineral specimens with care.
Internet Links for Kornelite
mindat.org URL:
https://www.mindat.org/min-2253.html
Please feel free to link to this page.
Please feel free to link to this page.
Search Engines:
External Links:
Mineral Dealers:
References for Kornelite
Reference List:
Merwin, H. E., F.Posnjak, (1937) Sulphate incrustations in the Copper Queen Mine, Bisbee, Arizona. American Mineralogist, 22 (5) 567-571
Cesbron, Fabien (1964) Contribution à la Minéralogie des sulfates de fer hydratés. Bulletin de Minéralogie, 87 (2) 125-143 doi:10.3406/bulmi.1964.5721
Localities for Kornelite
Locality List
 - This locality has map coordinates listed.
- This locality has map coordinates listed.
 - This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
- This locality has estimated coordinates.
ⓘ - Click for references and further information on this occurrence.
? - Indicates mineral may be doubtful at this locality.
 - Good crystals or important locality for species.
- Good crystals or important locality for species.
 - World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
- World class for species or very significant.
(TL) - Type Locality for a valid mineral species.
(FRL) - First Recorded Locality for everything else (eg varieties).
All localities listed without proper references should be considered as questionable.
Australia | |
| Sielecki (1988) |
| Sielecki (1988) | |
| R Bottrill |
Chile | |
| XRD and Wet Chemical by Gerhard Möhn |
Germany | |
| Walenta (1992) |
Greece | |
| Rieck et al. (1999) |
| Branko Rieck collection | |
| Branko Rieck collection |
Iran | |
| Khorasanipour et al. (2011) |
Japan | |
| Miura et al. (1994) |
Slovakia (TL) | |
| Palache et al. (1951) +1 other reference |
Spain | |
| Calvo (1999) |
USA | |
| Anthony et al. (1995) |
| Merwin et al. (1937) +3 other references |
| Van Nostrand Reinholt Press: 273 +1 other reference |
| |
| Anthony et al. (2016) |
| Van Nostrand Reinholt Press: 273. +1 other reference | |
| www.mineralsocal.org | |
| Hasenmueller et al. (2005, March) | |
| Mineralogical Record: 17:315 |
| Majzlan et al. (2011) |
| Van Loan et al. (1959) +1 other reference |
| Min News 6:8 p3 |
| Schindler et al. (2003) | |
| Patrick Haynes. ID via the late Howard ... |
| Palache et al. (1951) +1 other reference |
| Dietrich (1990) |
Quick NavTopAbout KorneliteUnique IdentifiersIMA Classification Classification Mineral SymbolsPhysical Properties Optical Data Chemistry Crystallography Crystal StructureX-Ray Powder DiffractionGeological EnvironmentType Occurrence Other LanguagesCommon AssociatesStrunz-MindatOther InformationInternet Links References Localities Locality List





 symbol to view information about a locality.
The
symbol to view information about a locality.
The 



Coso Hot Springs, Inyo County, California, USA